Abstract
AM and SML contributed equally as co-first authors.
KJ and DK contributed equally as co-senior authors.
Background Acute graft versus host disease (aGVHD) is a common complication of allogeneic hematopoietic stem cell transplant (alloHCT) and is associated with significant morbidity and mortality. Steroid refractory aGVHD (SR-aGVHD) carries a particularly grim prognosis. Ruxolitinib has shown promise for treatment of SR-aGVHD in phase 2 and 3 trials; however, safety and efficacy data outside of the clinical trial setting is lacking. We performed a multicenter retrospective study to examine the response to ruxolitinib and its efficacy in patients with SR-aGVHD in a Canadian population.
Methods and Patients This multicenter retrospective study includes patients that underwent alloHCT between 2015 and 2021, across five Canadian transplant centers in Calgary, Toronto, Vancouver, Quebec City, and Saskatoon. Patients were included in the study if they developed at least overall grade 2 aGVHD, met the criteria of SR-aGVHD, and were started on ruxolitinib for SR-aGVHD. Data was recorded on standardized forms by independent reviewers across the five sites. Survival outcomes were calculated by Kaplan-Meier. Treatment failure was defined as 1) treatment switch due to no response/no benefit, 2) non-relapse mortality (NRM), 3) relapse of primary disease, or 4) intolerance leading to discontinuation. Failure-free survival (FFS) was calculated from ruxolitinib start until the event of treatment failure or latest follow-up, while overall survival (OS) was calculated from the day of starting ruxolitinib therapy until death or latest follow-up.
Results We reviewed 59 patients treated with ruxolitinib for SR-aGVHD. The median age at time of ruxolitinib therapy was 53 (range 21-70) years, and 54.2% of patients were male. The most common indication for alloHCT was acute leukemia (48.2%), followed by myeloproliferative disorder/myelodysplastic syndrome (28.8%), lymphoma/chronic lymphocytic leukemia (18.6%), and others (5.1%). Approximately half of patients were treated with myeloablative conditioning (50.8%). Donor type included matched unrelated (44.1%), matched related (23.7%), mismatched unrelated (15.3%), haploidentical (15.3%) and cord blood (1.7%). The median onset of aGVHD was 65 days post alloHCT (range 15-265). Prior to initiation of ruxolitinib, most patients had overall grade 3 aGVHD (57.6%) and 16.9% had overall grade 4 disease. Patients had a median Karnofsky Performance Status (KPS) of 80% (range 30-100%) prior to ruxolitinib initiation.
A total of 36 patients (61.0%) obtained a complete response (CR) or partial response (PR) at 28 days. At day 56, 31 patients (52.5%) obtained a CR or PR. Patients that achieved a CR or PR at day 28 had a higher survival rate (69.2%), compared with patients that did not (31.6%) (p=0.037).
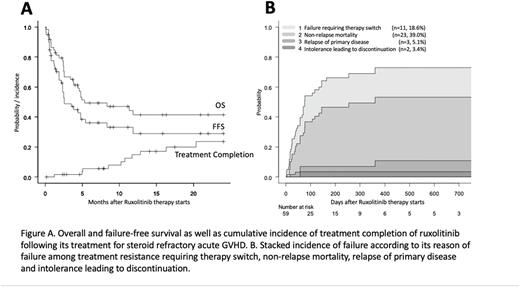
In terms of long-term outcomes, with a median follow-up of 391 days, OS at 12 months was 41.5% (95% CI, 27.6-54.8%), with a median OS duration of 5.3 months (Figure A). The 12 months' FFS rate was 29.1% (95% CI, 16.1-43.3%), with a median FFS of 2.6 months (Figure A). Reasons for failure included NRM (39.0%), need for therapy switch (18.6%), relapse of primary disease (5.1%), and intolerance (3.4%; Figure B).
Within 12 months after starting ruxolitinib, 14.8% of surviving patients were able to successfully taper off without flare of their aGVHD. By 24 months, 23.6% of surviving patients were able to taper off glucocorticoids (Figure 1A).
Glucocorticoid dose decreased over time with a mean daily prednisone dose 1.486±0.072 mg/kg at day 0 of ruxolitinib, compared to 0.152±0.038 mg/kg after 6 months (p<0.001).
Nineteen patients (32.2%) had an initial ruxolitinib dose of 10 mg twice daily (BID) while 37 (66.1%) were started on 5 mg BID, then escalated to 10mg BID within 3-7 days per the REACH1 study. There was no significant difference in OS observed between patients started on 10 mg BID compared to 5 mg BID.
Conclusions In this Canadian real-world experience-based study, we observed similar response rate and FFS rate that was found in the REACH2 trial for SR-aGVHD treated with ruxolitinib. We also observed that NRM was the primary reason for treatment failure, and saw that ruxolitinib therapy for SR-aGVHD enabled successful tapering of prednisone. Overall, these data support clinical trial evidence for ruxolitinib as useful therapeutic option for SR-aGVHD.
Disclosures
Elemary:BMS: Membership on an entity's Board of Directors or advisory committees; JAZZ: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. White:Novartis: Honoraria. Lemieux:AbbVie: Consultancy, Speakers Bureau. Kim:Sanofi: Consultancy, Honoraria; Merck: Consultancy; Paladin: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; BMS: Research Funding; Novartis: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal